Our pipeline of novel oral peptide candidates is strategically focused on addressing diseases with significant unmet patient needs, where we believe oral peptides can deliver enhanced therapeutic benefits relative to existing treatments.
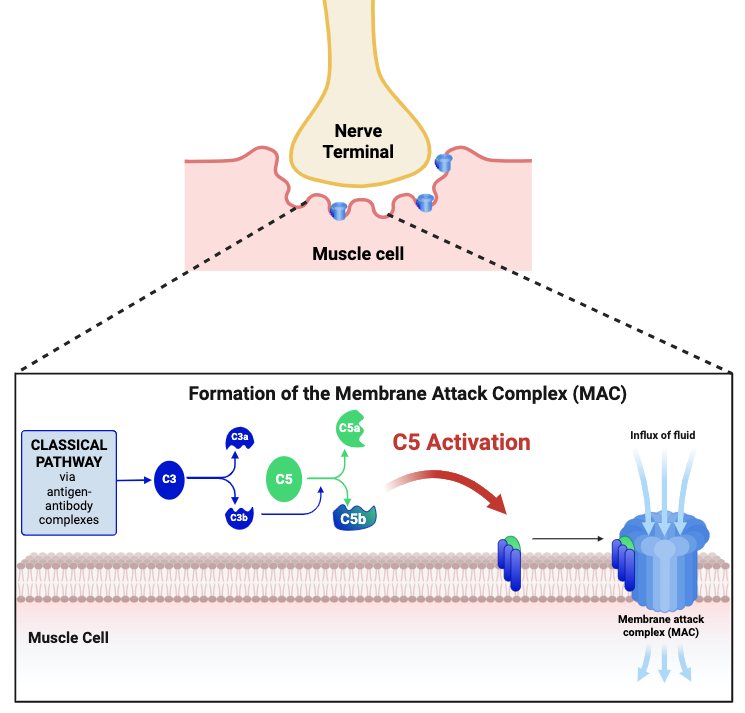
Generalized Myasthenia Gravis (gMG) is characterized by autoantibodies targeting acetylcholine receptors (AChRs) at neuromuscular junctions, which impairs signal transmission and causes muscle weakness. Autoantibodies activate the classical complement pathway, leading to C5a activation and assembly of the membrane attack complex (MAC). C5 is a critical component in the activation of the terminal pathway of the complement system. By binding to C5, antagonists inhibit its cleavage into C5a and C5b, thereby preventing the formation of the membrane attack complex (MAC). C5 inhibitory antibodies and peptides have been approved for the treatment of gMG; however, they are inconvenient for patients as they require frequent infusions or daily injections, respectively.
We are developing an innovative oral C5 inhibitor engineered for optimal potency, gastrointestinal stability and extended half-life. These properties of our proprietary compound will translate into enhanced efficacy and a compelling oral solution for patients seeking more accessible and convenient therapies.
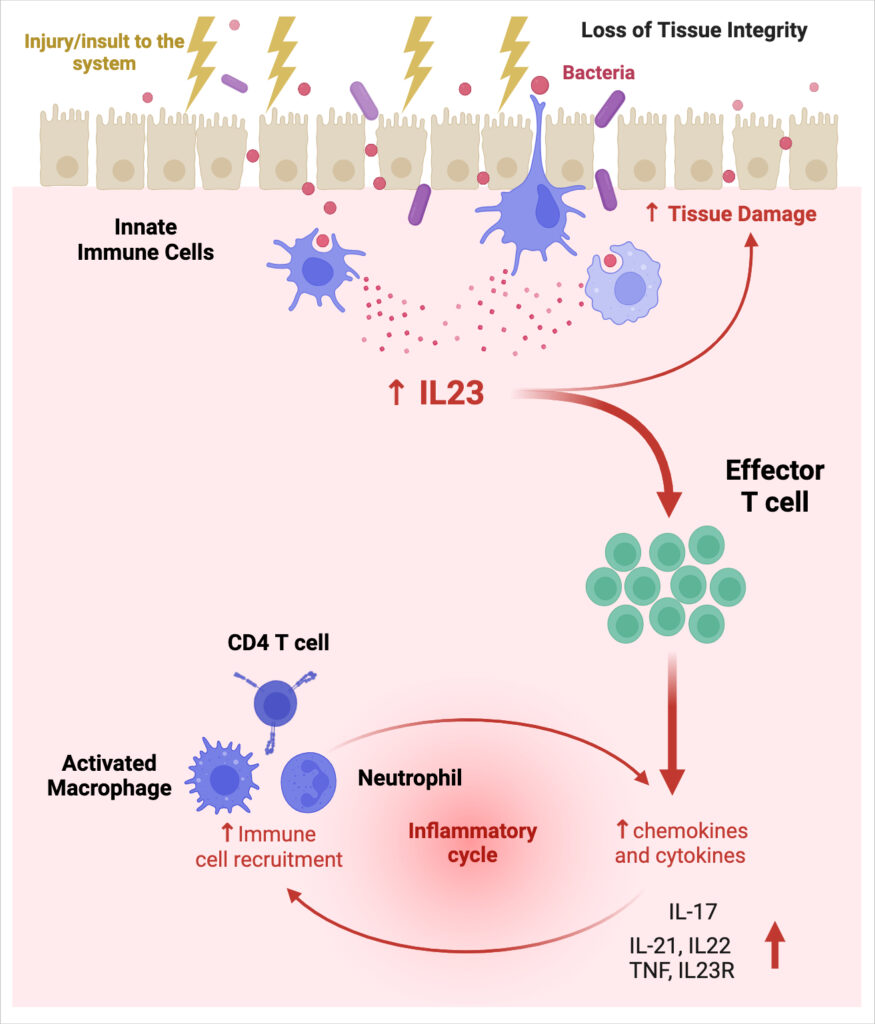
IL-23R is a receptor involved in the IL-23/IL-17 immune pathway, crucial in the pathogenesis of psoriasis and IBD. Upregulation of IL-23R expression is associated with increased susceptibility to IL-23 signaling, amplifying inflammation and immune responses in psoriasis and inflammatory bowel diseases (IBD), and underpinning the significant clinical efficacy of IL-23 blocking antibodies in these diseases. IL-23R antagonists block the interaction between IL-23 and its receptor, inhibiting the Th17 inflammatory pathway, cytokine production and IL23R upregulation, alleviating inflammation.
We are developing an innovative oral IL-23R antagonist by combining potent activity with gut stability and extended half-life. This medication will offer a more effective oral therapy option for patients seeking less invasive, more effective and convenient treatments.
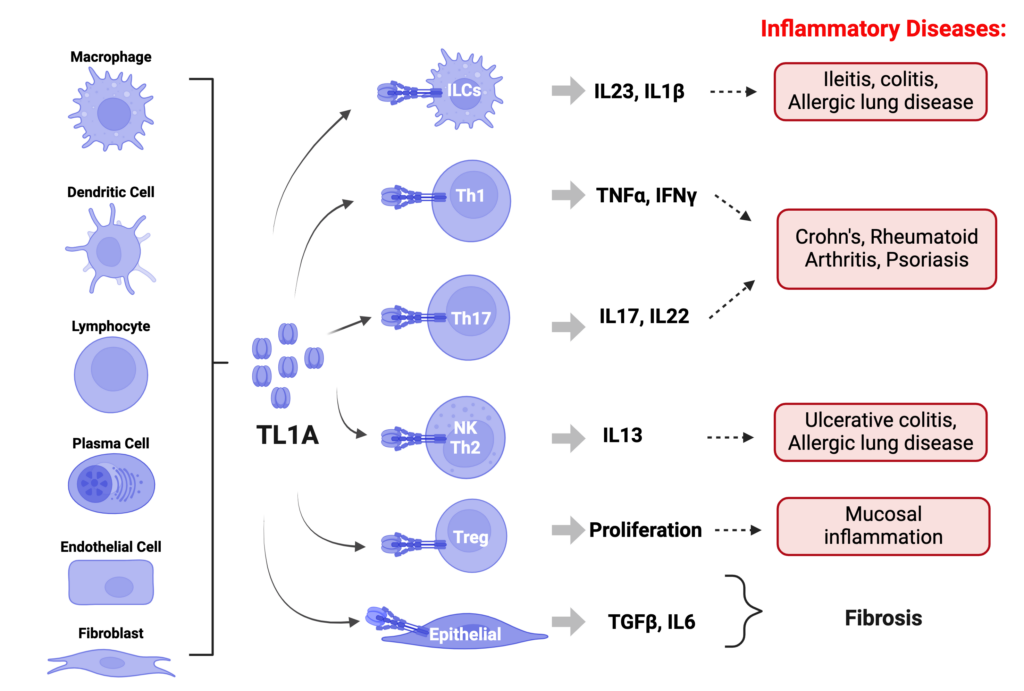
TL1A (TNF-like ligand 1A), also known as TNFSF15, is a member of the tumor necrosis factor (TNF) superfamily. TL1A plays a crucial role in regulating the immune system via interaction with its receptor DR3 on multiple cell types, promoting their proliferation and inflammatory cytokine production. Enhanced TL1A-DR3 signaling has been associated with several inflammatory conditions, including psoriasis, rheumatoid arthritis, and asthma, as well as inflammatory bowel diseases (IBD). In these diseases, overexpression of TL1A or DR3 contributes significantly to the inflammatory pathology by sustaining the activation of immune cells and the continuous production of inflammatory cytokines. TL1A blocking biologics have demonstrated efficacy in Phase 2 trials in patients with IBD.
We are developing an innovative oral TL1A inhibitor optimized for potency and gut stability; our drug will offer an extended half-life and enhanced efficacy, designed to meet the needs of patients seeking less invasive and more convenient therapy options.